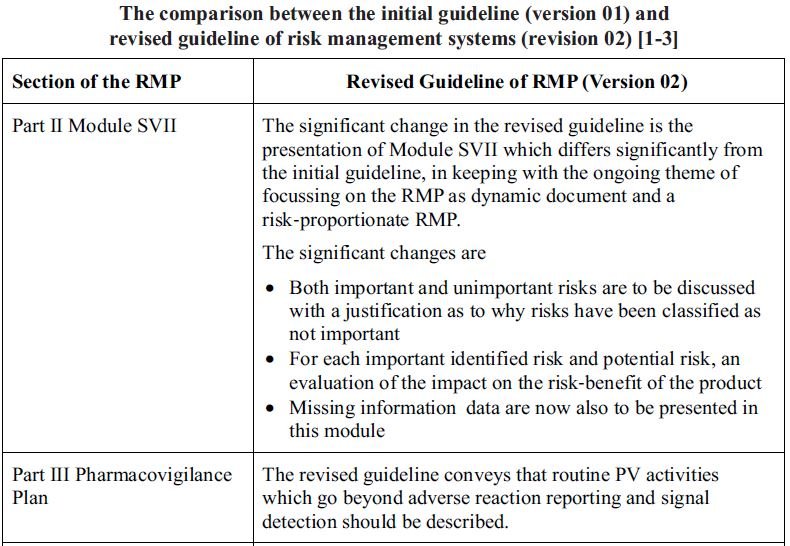
Introduction
The European Medicines Agency has revised the Good Pharmacovigilance
Practices (GVP) Module V on Risk Management Systems. The revised module (Revision 02) is effective from March 31, 2017. These revisions to the GVP Module V are intended to provide a more concise and clear description of risk management and how safety risks evolve through a product’s lifecycle based on the evidence from a variety of sources. The guidance is updated in parallel to an amended Risk